Good reasons for using remote control in the OR and medical practices
Nearly all of the latest steute Meditec projects exploit the advantages of wireless technology – in areas such as ophthalmology, neurosurgery, surgical microscopes and imaging techniques like CT and MRI. Examination couches and dental chairs are also increasingly controlled without cables. There are good reasons for this. Foot controls which are not connected by cable can be positioned more flexibly, facilitating more ergonomic operation of the medical device, and they are also more hygienic. The increased quality of a system incorporating a wireless user interface should not be underestimated. Remote control is a state-of-the-art technology which can help medical device manufacturers to set themselves apart from the competition.

More than thirty years of skilled development
In 2000, steute Meditec developed its first wireless foot control to run on the 868 MHz frequency band. It was for a magnetic resonance imaging (MRI) device which would be used in a shielded MRI room, making this technology a reliable and unproblematic choice. Other medical applications, however, demanded a frequency band that was freely available for other environments all over the world. That same year, a decision was made to implement the Bluetooth standard. By 2003, steute had developed its own wireless protocol suitable for medical devices. Today, steute controls using this technology can be found all over the world, with reliable signal transmission guaranteed by bidirectional communication and frequency hopping.

A wireless technology specifically for medical applications
Some extra development was still needed for the wireless systems, however, for example with regard to power consumption. This is why, in 2008, steute Meditec presented a new wireless technology developed from the outset specifically for medical applications, featuring very low power consumption, yet with reliable transmission and excellent coexistence. This technology was developed further in 2017, resulting in the version which is currently available. This SW2.4LE-MED wireless technology from steute features maximum energy efficiency in conjunction with increased transmission performance. It is used in both the standard steute Meditec products and its customised controls.
Typical application fields

Moving towards the interoperable OR
In collaboration with research institutes and manufacturers of medical devices, steute Meditec is currently working on research projects which also feature wireless communication. One goal here is the interoperable OR, in which several medical devices can be controlled using a single user interface.
Safe wireless technology SW2.4LE-MED
State-of-the-art wireless standard for medical equipment
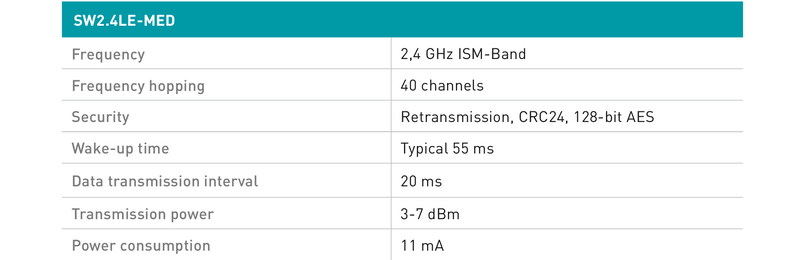
Signals are transmitted to the medical device via the third generation of steute wireless technology, featuring reduced power consumption in conjunction with high and configurable transmission performance. The response time is just 20 milliseconds.
The low-energy wireless technology in the latest steute user interfaces enables conventional alkaline batteries (e.g. AA or C) to be used instead of lithium ion rechargeable batteries. Eliminating rechargeable batteries reduces costs by removing the need for charging devices and charging management. The latest steute Meditec wireless technology works on the 2.4 GHz frequency band, available worlwide and free of charge.
Low power consumption – short connection times
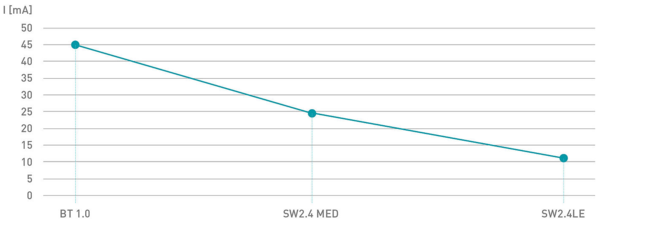
One of the features of the new, third-generation SW2.4LE-MED wireless technology from steute is an extremely low power consumption during active operation of just 11 mA. Thanks to a "sleep" mode, which the wireless system enters after a preconfigurable time lapse, the power consumption is reduced to almost nothing. The shift back from the resting mode to the active mode takes just 50 to 60 milliseconds and goes unnoticed by the user. The entire process chain takes place within this very short period: foot control actuation, "waking up", signal transmission, signal reception, signal processing and switching of the output signal.
Higher and configurable transmission performance
While the power consumption of the wireless technology is lower, the transmission performance is higher than that of the second generation, and also flexibly configurable. This means that e.g a user interface controlling an X-ray device can send signals from a neighbouring control room. Clear assignation of foot control to receiver via the »pairing« procedure prevents erroneous operation and facilitates interference-free operation of multiple wireless user interfaces inside one OR.
International standards implemented
Transmission safety of the new SW2.4LE-MED is documented through a very low residual error probability of below 1 x 10-9 1/h. The wireless system hereby meets the requirements of SIL 3 (Safety Integrity Level to IEC 61508). A further advantage is its improved 128 bit-AES encryption, which also meets the security requirements of the FDA.
Powered by commercially available, not by rechargeable batteries
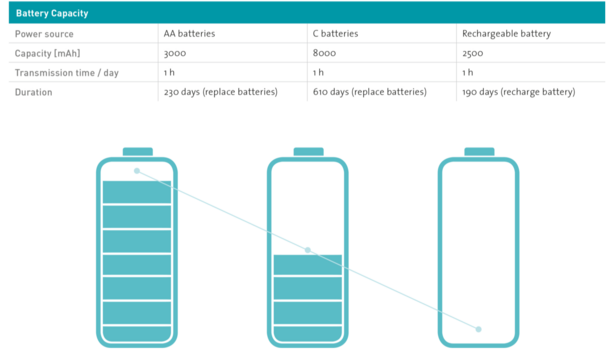
The low power consumption means that commercially available batteries can now be used instead of rechargeable batteries, eliminating charging devices and charging management. With conventional medical applications, the battery lifetime exceeds one year, so that device manufacturers can replace them as part of their annual service. The battery status is displayed at all times. Users can change the batteries with no need for tools: the waterproof battery compartment is opened by a quarter-turn of the knob. The protection class of the foot control (IP X8) remains unaffected.
Non-tiring and intuitive operation
In addition to optimised power supply and high transmission reliability for these foot controls, the steute engineers also attached great importance to an ergonomic design facilitating non-tiring and intuitive operation. Optional push buttons can also be actuated by foot. A folding bar enables controls to be positioned easily, while the wireless actuation gives users additional freedom of movement without the restrictions of a cabled solution, which can also be problematic for hygienic reasons.
Compliant use of state-of-the-art user interfaces for medical devices
Documentation requirements for radio systems in the OR
New directives and standards, including the EU RED directive and the modified EMC directive, are increasing the levels of testing and documentation required during the development of medical devices, especially in connection with wireless technologies. This also impacts country-specific regulations regarding the use of radio equipment.

Coexistence with other radio networks
The stricter requirements for medical equipment are due to the increasing variety of wireless systems and the very high standard of safety essential to this field. Consequently, the directives play a major role in guaranteeing the coexistence of different radio systems, such as Wi-Fi, Bluetooth, Zigbee and microwaves. These inspections must comply with the relevant standards (IEEE / ANSI C63.27), and the results must be documented.
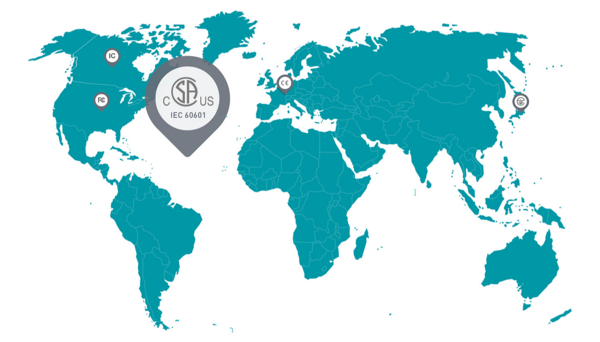
Approvals for all major markets
Our “Classic” range of user interfaces makes it easy for customers to produce the required evidence of conformity. These standard wireless controls have all been tested in accordance with the relevant directives, and the radio module is approved for major markets such as the USA (FCC), Canada (IC) and Japan (ARIB). The medical device documentation (including test reports) is supplied with our products and can be integrated in the documentation of the end device.
Testing and documentation for customised controls
The steute development team is experienced in the individual testing and documentation of “Custom” user interfaces. The goal is to eliminate as much of the testing and documentation work for our customers as possible. The wireless controls can additionally be provided with certificates of conformity with FCC (USA), IC (Canada), and MIC (Japan), as well as with test results proving compliance with the relevant directives.
"Simplified approval" for wireless controls
As the marketer of the medical device, the manufacturer is responsible for proving compliance. With the tests already conducted, the documentation and the test reports, the manufacturer ultimately has to ensure that the user interface has been integrated correctly within the overall system. This requires a "delta analysis" or "gap analysis", which is simpler, faster and cheaper. This procedure is known as "simplified approval" for wireless products in accordance with the ETSI directive (ETSI EG 203 367).
"All-inclusive": certificate for global approval
On request, steute can provide a "Certificate of compliance", issued by the CSA following an independent inspection. A "CB certificate" can also be provided, corresponding to the "CB scheme" for electromedicine and accepted by international approval bodies.
Support with software testing
Individual software solutions are created for our customised user interfaces, requiring comprehensive testing and documentation. Here, the phases of the life cycle process to EN 62304 provide orientation. A test specification is drawn up prior to the programming, listing the target outcome. After programming, the target outcome is then compared with the actual outcome. The software can only be released when all tests have been successfully completed. steute Meditec can perform all the necessary tests, shortening development times.
Wireless FAQs - The future starts at ground level
The next generation in medical device interfacing from the recognized world leader in medical-grade foot switches.
Answers to frequently asked questions and quantifiable benefits for you.